#008 Hydrogen Embrittlement - Plating Embrittlement
[Fig.1] shows a listing of hydrogen embrittlement rates of various plating processes. It is easily imaginable that possibilities of hydrogen embrittlement exists for electro-plating processes, which uses the work piece as a cathode electrode for the electrolysis. However, non-electrolysis process such as electroless nickel plating could also cause hydrogen embrittlement as well. Here, the hydrogen generation is thought to be caused by reducing agents used in place of the direct current used for electro-plating.
Also, in chrome plating where non dissolving anodes such as lead and graphite are used, unlike other plating processes where dissolving anodes (zinc, nickel, etc.), vigorous electrolysis of water takes place during redox of chromate ions dissolved in the plating bath in order to obtain the chromium metal. This vigorous electrolysis causes hydrogen generation.
![[Fig.1] Hydrogen embrittlement rates of various plating processes [Fig.1] Hydrogen embrittlement rates of various plating processes](http://www.misumi-techcentral.com/tt/en/surface/images/008_1.gif)
From [Fig.1] above, it can be seen that acidic plating baths such as nickel plating, nickel alloy plating, and chloride zinc plating have very low hydrogen embrittlement rates, but alkaline plating baths such as zinc cyanide bath and copper cyanide bath are more likely to cause hydrogen embrittlement. It is considered, in general, that most of the electrical current applied is used for electrolysis of water resulting in high hydrogen generation with the alkaline baths due to low cathode current efficiency, as opposed to acidic baths where the current is mostly used for redox of metal ions due to high cathode current efficiency.
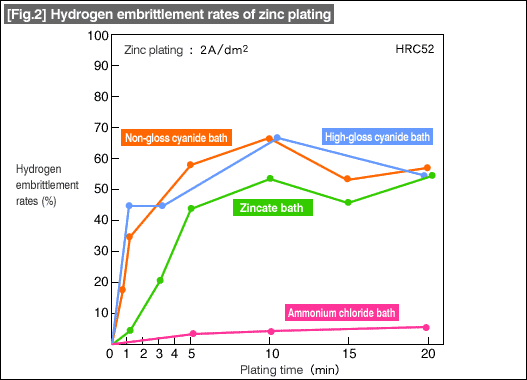
Hydrogen embrittlement rates of zinc plating in cyanide bath (alkaline) and chloride bath (acidic) are shown in [Fig.2] below.
- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



