#147 Principle of Electroplating
How are the electroplate coating created? Simple explanation of the principle is as follows.
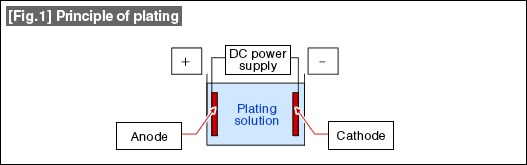
As shown in [Fig.1], the product to be plated is placed in a solution containing the ions of the plating metal as the cathode (-) ,and with an anode (+) made of the plating metal (soluble anode), then an appropriate DC voltage is applied from a power supply between both poles. As a current flows, a reduction reaction occurs at the cathode and precipitates the plating metal and the plate coating grows.
| ※ | (Note 1) What is Soluble Anode? It is a type of anode that the metal dissolves in the plating solution as the process progresses. This supplements the consumed ions within the plating solution. If the precipitated plating metal at the cathode and dissolved metal at the anode are equal in the amounts, the metal amount in the solution does not vary and is ideal. (Copper plating, etc.) |
On the other hand, anodes that do not dissolve in the plating solution (Insoluble anode) is also used. In this case the metal ion is supplied by chemicals. (Chrome plating, etc.)
| ※ | (Note 2) What is Reduction Reaction? It is a reaction where the metal ions within the solution (the state which the metal is dissolved in the solution) turn into a metal on the surface of the cathode (product to be plated) by the DC current (electron) causing the ions to lose the electrical charge. An inverse of this is the oxidation reaction occurring at the anode, where the metal dissolves into the solution becoming the metal ions. Both of these cathode and anode reactions occur simultaneously. |
Typically, [Plating] points to the metallic coatings applied for ornamental and anti-corrosion purposes in Japan, although the word sometimes also points to the process of applying the plate coatings itself. For instance, "This Plating is hard" would mean that the plate coating has high hardness, but "This Plating is awful" would point to the entire plating process including pre-plating polishing as well as the post plating processes.
- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



