#207 Hot Dipping
Hot dipping is a surface treatment method used to form a film of molten metal on a product surface by immersing the product into a bath of molten metal at a high temperature and lifting it out after dipping.In Japan, it has long been a familiar method known as tempura plating from how the film is formed.Although the principle is simple, this method requires highly technical skills in order to make products that meet a certain quality standard.
| Various metals are used for hot dipping, such as zinc, aluminum, lead, tin plating, and composites of these metals. The most frequently used method is hot dip galvanizing, which is applied for a wide range of steel products, including various tubes, plates, wire ropes, metal meshes and more. In recent years, the industry has shifted its emphasis from a heavy, thick, long, and large structure to a light, slim, short, and compact structure. Owing to this industry trend, hot dipping rarely receives the attention it deserves, but it supports the industrial infrastructure by being used as a heavy-duty anticorrosion measure for transmission towers, large-scale bridges, and marine hardware. |
 |
Only the metals with a lower melting point than the substrate can be used for hot dipping. However, hot dipping is frequently used in corrosive environments that require a high degree of anti-corrosion protection (zinc plating) or high-temperature oxidation resistance (molten aluminum plating) because hot dipping can form a relatively thick metal film.
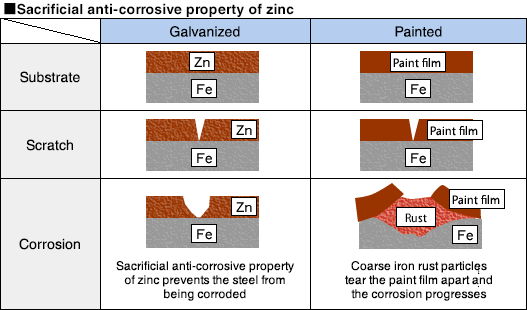
Hot dip galvanizing provides highly reliable corrosion resistance because of the property of zinc being electrochemically less noble than steel. Even when the substrate is scratched, a local battery is formed between the substrate metal and the plating metal where zinc serves as the sacrificial anode and protects the steel substrate from being corroded.Thus, the thicker the plating, the longer the product life.Because metals, such as tin/lead plating, being more electrochemically noble than steel will not serve as the sacrificial anode, the performance will be based on the anti-corrosive properties of the plating metals themselves.
- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



