#077 Corrosion Protection Measures - Anti-corrosives
In limited environments such as cooling systems for chemical plant systems, adequate amounts of anti-corrosive use to prevent metal corrosions is possible. The environments where the anti-corrosives can be effective are with water, acids, and humidity, and mainly for steel. There are some anti-corrosive intended for use with copper and copper alloys but in limited applications compared to steel.
The mechanism of anti-corrosives vary depending on the chemical and can be classified into the following three.
(1) Passivation Agents
Agents added to environments to promote passivation in order to prevent corrosions of metal surfaces is called Passivation Agents. Steel has a self-passivating nature in water but not in normal water containing air. Steel forms a passivated layer in water only with existence of stronger oxidant. Representative passivation agents of this type are chromate and sodium nitrite.
These agents are added to the recirculating cooling water at 50~100ppm, and evaporation latent heat at the cooling tower cools the system. However, chromate is no longer used due to recent environmental concerns on splashes and waste disposal problems.
(2) Corrosion Protection Layer Formed on Steel Surface
This is a corrosion protection method of forming layers with anti-corrosive agent itself or compounds of the agents and waterborne elements. When phosphate polymer and phosphonate are added to water, they dissolve well but they bond with calcium ions and separately added zinc ions and form insoluble coating layer on the steel surface. These are thick and porous hydroxide or oxide layers unlike the passivated layers, and inhibit corrosions by preventing diffusion actions of dissolved oxygen onto the steel surfaces. These agents are now used for open cooling systems in place of chromates.
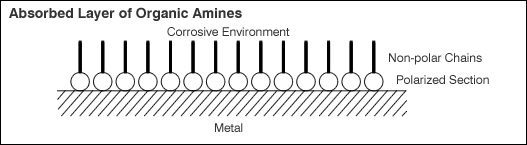
(3) Layer Forming by Steel Surface Absorption
This is a molecular layer forming process by agents being absorbed by the metal surfaces. Typical example pf this is organic amines. The polarized section of the amines containing nitrogen adhere to the steel surface, and other non-polarized sections are oriented outward creating a water repelling structure. When the surface is covered with such absorbed layer, the overall electrical potential will become positive, and frequently used as an inhibitor (anti-dissolve agent) for pickling baths.
- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



