#081 Corrosion Protection Measures - Corrosion Resistant Paints
Simply using the previously discussed corrosion resistant metals for structural materials in order to protect from corrosions will quickly become very expensive. Paints are used to protect from electrolytic and chemical corrosions by physically isolating the subject metals by surface coating with organic materials such as plastics.
Painting is applied on the subject metals by spraying, brushing, and roller coating. Subsequently, strong paint coats are formed by chemical polymerization of the ingredients. Some paints require 120 degrees C or higher temperature heating for the curing processes but natural curing paints are used for large structures such as steel beams, etc.
[Table 1] shows the general characteristics of corrosion resistant paints used in chemical plants and plating facilities.
[Table 1] General Characteristics of Paints
|
| ※ | Note : 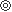 Excellent, Excellent, 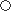 Good, Good, 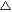 OK, OK, 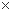 Not Applicable Not Applicable |
- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



