#164 Plate Coating Thickness Management - Principle of Plating
Plate coating thickness management
The most importantly viewed aspect of plating coat quality control is the coating thickness management. Historically, troubles such as ending up with an 8μm thick coating despite 10μm zinc plate coating was what was ordered, etc. have been occurring endlessly. Here, let us take a look at how plate coating thickness is managed.
Principle of plating
(1) Principle of electroplating
| In general, electroplating is performed as shown in the [Fig.1], where the product to be plated is made into an anode submerged in the plating bath, and cathode is the plate metal (i.e. nickel for nickel plating). A DC current is applied on them to plate. The following reaction is occurring at the poles. | 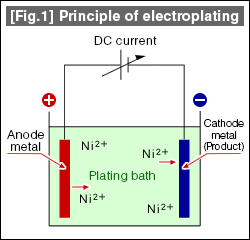 |
|
At the cathode, the metal ions in the bath Mn+ (n is the valence of the metal ion, n=2 for nickel plating) acquires n electrons (e) becoming metal, and deposition on the product surface occurs. On the other hand, at the anode, the anode metal in emits n number of electrons and dissolves the bath, becoming metal ions (Mn+). By continuing to apply the current to the plating bath, this reaction takes place continuously and the plating thickness grows gradually. The plating process is halted when a desired plate thick is reached. The important fact here is that the energy forming the plate coating is the "DC current" used for the reduction reaction of metal ions. DC power is represented by Current (amps) x Time (min.), and the plating thickness is affected by the current and time per one product. This indicates that targeted plating thickness can be obtained in short amount of time by applying a large current, though it is not unlimited. There will be some limitations due to the plating bath types and shapes of the products to be plated. In general, a concept of "Current density" is used to determine the strength of the electrical current. This indicates the strength of current applied per unit area of the product, and typically represented with A/dm2 (Amperes per 1 square decimeter 100x100mm). Therefore, surface area of the product to be plated is calculated, and the applicable current density multiplied constitutes the current (A) to be applied per one product.
- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



