#052 Passivation of Metals
There are some metals far more chemically stable than can be judged by the metal activity sequence shown in [Fig.1] (Ionization Tendency). Those are: Aluminum, Nickel, Titanium, Chrome, and Molybdenum. These metals are not necessarily located near the Superior side within the active metal series. Titanium is located between Magnesium and Aluminum, and Chromium and Molybdenum are at somewhat above Iron.
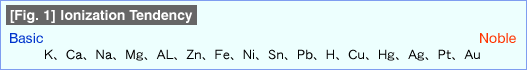
The reason these metals are chemically stable is that they tend to form a layer called "Passive Layer" on the surfaces. Passive layer is a type of oxide, and is several nanometers thick, not visible to eyes. Therefore, the metal retains its original metallic luster after forming the passive layer. This layer is instantly formed when the metal is exposed to an environment. The layer becomes more stable as the time elapses, but notably this layer, even thin, possesses the chemical stability immediately. Metals gaining the passive layers is called "Passivation", and the layer coated state is called "Passivated".
The most notable characteristic of metals being passivated is that they will sustain zero corrosion. Another characteristic is that passivated metals do not act accordingly to the metal activity sequence (Ionization Tendency) but will act rather like they belong in the "Noble" region of the metal activity sequence.
When two different metals are immersed in sea water while in contact, one side becomes of a positive pole, the other an negative pole, and the negative pole side will corrode. Characteristic more likely to be polarized positive is called Noble, and more likely to be polarized negative is called Basic. The metals more easily forming passive layers, such as Chromium and Titanium, will have Noble characteristic by creating layers of passive film compared to the states without the films.
The only metals that form passive layers are the ones aforementioned, and alloys that contain them as the main constituents. However, these metals and alloys depend on the environments as to if they will form the passive layers or not.
Most of these metals will become passivated in water containing dissolved oxygen. In hydrochloric and dilute sulfuric acids the passivation occurs if the acidities are very low, but the passive layer will be dissolved if the acidities are high.
The passive layer is stable in oxidizing acid such as Nitric Acid, as it is in alkaline solutions. In highly oxidizing acids such as concentrated nitric acid and concentrated sulfuric acid, iron will easily become passivated.
Stainless steel is an alloy that easily becomes passive since it contains much Chromium. When stainless steel is in neutral water containing dissolved oxygen, it is protected by a passive layer. But when in water rich with Chlorine Ions such as sea water, the passive layer will experience localized destructions and small but deep pitting corrosions will result.
- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



