#091 Electrolytic Color Treatment
The previous 88nd natural coloring of anodic oxidation film was described as the combination of the aluminum alloy, the electrolytic bath, and the waveform of the power supply to provide the different colors. I will now explain the coloring method by first performing anodic oxidation treatment and then electrolysis again. This method is called the secondary electrolytic coloring method because electrolysis is performed twice.
In general, a sulfuric acid bath forms the initial oxidation film. The reason is because the silver-white film can be generated at low cost.
Aqueous solutions of Ni, Sn, Cu, and Ag are used in the next electrolytic coloring bath, and the color tone varies depending on the metal.
The principle of electrolytic coloring is to electrochemically (in the same way as electroplating) precipitate metal or its oxide in the pores of the oxidation film to produce colors. The color tone changes from light to dark depending on the amount of precipitation.
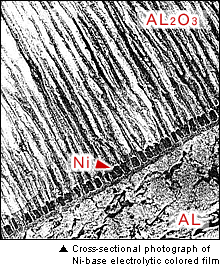
The most frequent users of electrolytic coloring in our country are building material manufacturers, thus you see window sashes, entrance doors, roofs, and fences, for example, that have been treated. In general, bronze is the most popular color, and a transparent electrophoretic coating is added after coloring to increase the corrosion resistance of the building materials.

- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



