#248 Metal Cleaning - Surfactant - 1
The desired effect of water-based wet metallic cleaning will not be achieved without the surfactants. A small amount of surfactants added to acid pickling, alkali degreasing, or activation bath enhances the performance of removing dirt and smudges on metal surfaces through various actions, including infiltration, adsorption, emulsification, suspension, dispersion, foaming, and surface tension reduction.
Surfactants do not play a leading role like acid that dissolves oxide films or organic solution that removes oil content by dissolving, but they help to create conditions where such reactions are likely to occur.
(1)Types of surfactants
(1)Anion surfactant
Soap is the typical example of this surfactant. It is used in synthetic detergents such as ABS (Alkyl Benzene Sulfonate). For sodium alkyl benzene sulfonate, the strong cleansing performance is achieved when alkyl benzene sulfonic acid dissociates into a negatively charged anion and Na dissociates into a positively charged cation in an aqueous solution.
(2)Cationic surfactant
It is negatively charged in water. Primary, secondary, and tertiary amines are available.
(3)Non-ionic surfactant
Non-ionic surfactants are substances containing polar groups that do not dissociate to ionic species in aqueous solution. Only nonylphenol type is used as industrial detergents. The cleansing power is comparable to that of anion surfactants. The advantage is that the cleansing performance will not be degraded even when used in an acidic solution.
(2)Properties of surfactants
(1)HLB (Hydrophile-Lipophile Balance)
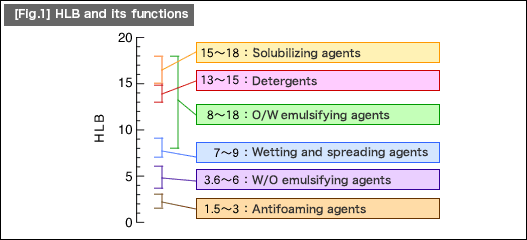
Surfactants have a structure comprised of lipophilic group (reaction group easily bonds with fat) and hydrophilic group (reaction group easily reacts with water). The HLB is an indicator of these balance. [Fig.1] shows the HLB and its functions.
An HLB value of zero corresponds to the most lipophilic molecule, and an HLB value of 20 corresponds to the most hydrophilic molecule. All surfactants are placed between these values, and each value represents its respective property.
Surfactants with an HLB value of five or less will be emulsified and dispersed into water without being dissolved. With an HLB value of 10 or more, surfactants appear to be dissolved in water as it produces a clear solution. However, at this time, most of the surfactant molecules aggregate in water as micelles.
"O/W emulsifying agents" in the figure is a state where oil is dispersed in water. Milk is a typical example of this type (milk fat is dispersed into water). "W/O emulsifying agents" is a state where water is dispersed into oil, as in butter or margarine, for example.
- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



