#242 Atomic Absorption Analysis
(1)Measurement principle
This is an official analysis method adopted by JIS as well. By placing atoms in a high-temperature flame (some methods do not involve the flame), the outermost electron of all the electrons surrounding the nucleus will be excited to a higher energy level. When they return to the ground electronic state after a short period, each element emits light at a characteristic wavelength of the corresponding atom. This process is called "atomic emission".
On the other hand, when you emit specific light from the outside to this atom, the light will be absorbed by the atom. This process is called "atomic absorption". The degree of light being absorbed (absorbance) is an indicator of the corresponding atom's concentration.
(2)Measurement device
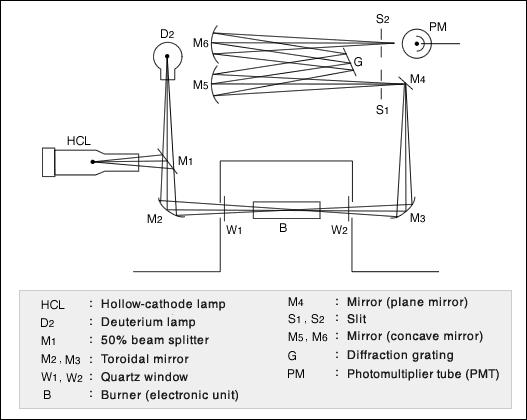
The figure here is an example of the optical system for an atomic absorption photometer. Use an appropriate type of a hollow-cathode lamp (HCL) for the atom absorption wavelength you are going to measure. The deuterium lamp (D2) is used for background correction. Substances such as hydrogen, acetylene, and nitrous oxide are used for the heat source of the burner (B) to bring atoms to the excited state. Inject the sample in a mist form into this burner. The light passed through the burner flame is converted into an electric signal by the photomultiplier tube (PMT), which will be displayed and printed out as the concentration data.
(3)Characteristics
Although this method can analyze most of the metallic elements, a pretreatment process is required prior to analysis. Unlike the fluorescence X-ray analysis introduced in the previous volume, this method is not designed for analyzing samples in the solid state.
The preparation method may vary product to product based on the materials, but it is necessary to prepare the liquid sample for measurement by dissolving the target element using strong acid like "agua regia" and then diluting it at an appropriate multiplying factor. Since the sample's concentration is measured in units of single-digit ppm, the technological skills of diluting samples by adding pure water will determine the degree of errors.
- Environmental conservation
- Hot Dipping
- Anodic Oxidation Process
- Anodic oxidation treatment
- Anodizing
- Corrosion - Corrosion Protection
- Electroless Plating
- Electroplating
- Heat treating
- Hydrogen embrittlement
- Metal cleaning
- Metal etching
- Painting
- Special paints
- Surface Treatment
- Surface-treated steel sheets
- Thermal Spraying



