When metals are subjected to pickling processes, the metals are dissolved by the acids and hydrogen is generated. The hydrogen is also generated during electrolytic de-greasing, electrolytic pickling, and electro-plating. This hydrogen is occluded (absorbed) by the base metal, especially steel alloys and makes the steel brittle. This phenomenon is the Hydrogen Embrittlement. Parts with hydrogen embrittlement can break after being subjected to loadings. Here, we'll look into some testing methods to see how much hydrogen embrittlement there is on plated steel.
(1) Delta Gage
This is a method developed by Delta Research Co. headed by Mr. Takada of Takada Labs. The method uses steel plates made of alloys susceptible to hydrogen embrittlement, press bent in a constant speed vise, and the vise travel distance to the point of breakage is measured. The distance traveled indicates the degradation of flexibility of the test specimen, in turn indicates the extent of the hydrogen embrittlement. It is also called "Slow press-bent destruction method". Fig. 1 below shows the measurement principle of the Delta Gage method.
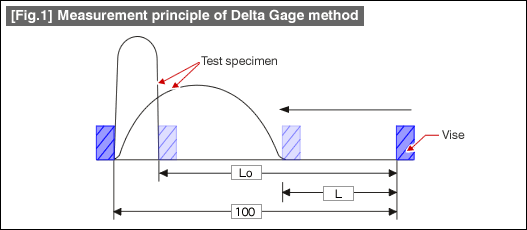
In Fig.1., consider that...
|
then...
|
For the test specimen: material; SK5 (C0.85%), shape; 9mm x 100mm x 0.8mm thick, hardened at 850 deg.C, tempered at 450 deg.C is used. The test is performed by placing the flat test specimen between the jaws of the vise, and slowly closing the jaws at a constant speed to bend the test specimen. If any hydrogen occlusion is present in the test specimen, the hydrogen will migrate diffusively towards the area of tensile stress concentration, and the specimen becomes brittle and likely to break in comparison to a specimen with no hydrogen occlusion.
As seen, the Delta Gage method can numerically establish the hydrogen embrittlement rates with easy operations, excellent for factory floors where the plating processes take place.
