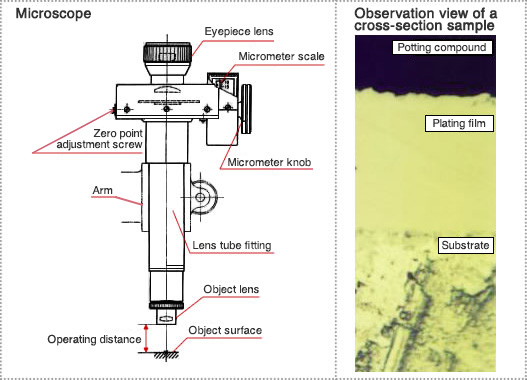
(1) Measurement principle
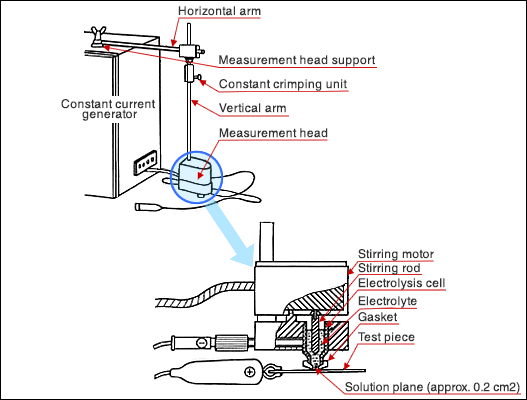
Set the electrolysis equipment (measurement head) on the plating surface. Pour the electrolyte and polarize a specified area of the plating surface. Electrolysis at constant current dissolves the plating. In this method, the plating thickness is measured by the electricity consumed for this process. The amount of metal dissolution (plating thickness) is proportionate to the time in the constant current dissolution. Therefore, the time required for electrolysis represents the plating thickness.
(2) Equipment
The measurement equipment is comprised of the constant DC current supply unit, electrolytic head (consisting of an electrolytic bath, a stirring device, retainers, etc.), and measuring unit.
In order to prevent liquid spill and to keep the electrolysis area constant, a gasket is installed at the bottom end where the plating surface contacts the electrolytic bath. A gasket for the 0.2cm2 area of electrolysis is generally used.
Since the electrochemical equivalent varies by the type of the plating metal, it is possible to select a constant current value unique to each metal.
(3) Electrolyte
| 1. | Because this test utilizes electrochemical reaction, you need to use an electrolyte that does not corrode the plating films while they are not energized. | |
| 2. | The anode current efficiency should be 100% for anode electrolytic treatments of plating surface. | |
| 3. | When a substrate is exposed after dissolution of its plating film (this phenomenon is referred to as "end point"), a significant change in electrolysis voltage must be observed. | |
| 4. | None of the electrolyte meets the above-mentioned conditions for all types of metals. For this reason, many types of electrolyte have been developed. It is important to choose an appropriate type of electrolyte according to the plating metal and substrate or by the combination of upper and lower plating metals for multi-layered plating. |