Because of the aesthetic appearance and anticorrosion performance along with superior resistance to sulfur and heat in particular, molten aluminum plating is a popular technique applied in both batch and continuous production systems.
(1)Plating
(1)Batch production
Batch plating will be applied for single item groups, such as tubing, rolled steel, cast and wrought products. The plating processes are shown in the below figure.
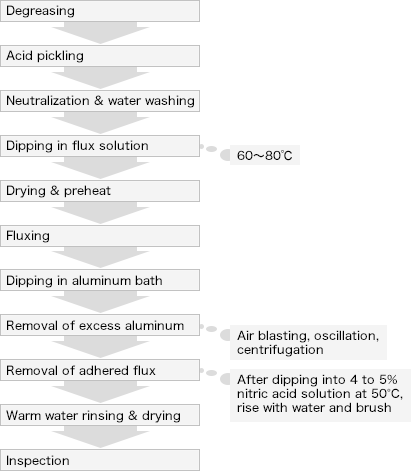
Since the plating bath temperature becomes as high as 700℃, the flux has been specially developed for this temperature. The flux is made primarily of chloride compounds, such as potassium chloride, and a mixture of fluoride compounds, aluminum fluoride for example. The figure shown here is a process of dry flux treatment. In addition, a wet-type system where the flux is melted on top of the plating bath is also available. AL-Si (Si 7.5 to 9.0%) alloy seems to be the common composition of the plating bath.
(2)Continuous production
The following figure is an example of the continuous production line where plating is applied on strip steel plates.
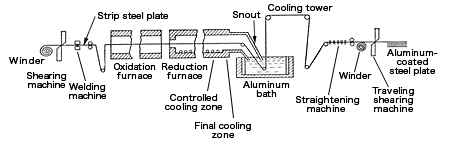
At 500℃, grease and organic substances on steel plate surfaces will be oxidized and combusted. In the next step, the steel will be annealed in the annealing furnace (850 to 900℃) filled with ammonia decomposition gas where a reduction occurs. In the cooling zone, the steel plates will be cooled down to the temperature of the plating. Without being exposed to air, the steel plates are plated in the aluminum-plating bath.
The snout in the final cooling zone of this figure is used for inputting alkali metals such as sodium, which will be evaporated to generate sodium aluminate on the plating bath surface. This substance inhibits the generation of aluminum oxides or nitrides and keeps the plating surface smooth.
![[Fig.1] Cr effluent treatment by ion-exchange resin [Fig.1] Cr effluent treatment by ion-exchange resin](/tt/en/surface/245.gif)
