(1) Basic process of Anodic Oxidation
Basic process of Anodic Oxidation is as follows.
Preliminary processes -> Pretreatments -> Anodic Oxidation process -> Post treatments
1. Preliminary process
A processes that effect the final finish of the coating such as buffing, brushed finishing, satin finish, and pattern marking that are generally performed outside of the actual anodizing process.
2. Preliminary treatment
These are: degreasing, etching, and desmut, etc that are cleaning and dissolving of the base surfaces. Inappropriate pretreatment causes stains and uneven finishes.
3. Anodic Oxidation process
This is a process to create the anodized coating. In order to obtain satisfactory coating quality, appropriate electrolyte bath, power supply waveform, bath temp., agitation, and process time are selected.
4. Post treatments
After the electrolytic process (anodic oxidation) the surface pore sealing is always performed. Before the surface is sealed, dying, secondary electrolytic coloring, and electrostatic coating are applied as need.
(2) Anodize process steps for various applications
The following are anodizing steps based on various applications. (Water cleaning omitted)
Construction Material (sash, spandrel, partition, door, fence, etc)
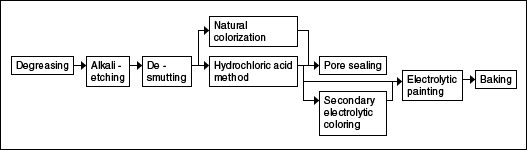
Industrial Products (Home appliance parts, heat exchangers, machine parts, etc.)
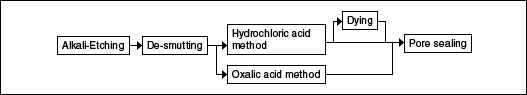
Cooking utensils (pots, kettles, warmers, etc.)
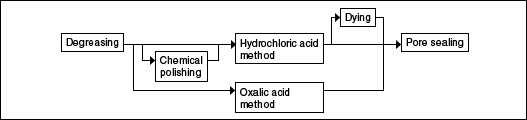
Ornamental products (automotive decoration parts, general decorative items, mirrors, name plates, camera parts, etc.)

![[Fig.1] [Fig.1]](http://www.misumi-techcentral.com/tt/en/surface/images/101_1.gif)
![[Fig.2] [Fig.2]](http://www.misumi-techcentral.com/tt/en/surface/images/101_2.jpg)
![[Fig.1] Anodize Process Diagram [Fig.1] Anodize Process Diagram](http://www.misumi-techcentral.com/tt/en/surface/images/086_1.gif)
![[Fig.2] Layer Formation Model Diagram [Fig.2] Layer Formation Model Diagram](http://www.misumi-techcentral.com/tt/en/surface/images/086_2.gif)
