(1)Steam with high dryness
In the previous volume, we learned that the steam generated from a boiler is classified into dry saturated steam, wet steam, and superheated steam. Among of all, dry saturated steam at 100% dryness shown in the steam table is the type of steam required in surface treatment processes.
Even if a boiler outlet supplies dry saturated steam, the dryness of steam decreases (the wetness increases) due to the heat loss along the way. When the dryness of steam decreases, the latent heat of steam also drops, which ultimately reduces the heating capacity.
In addition, the steam demand may surpass the amount of steam generation during the peak period, such as the preparation hour for daily operations. During such period, the boiler produces wet steam with less dryness.
Let's compare the latent heat of steam at varying dryness in [Table 1].
[Table 1] Difference of latent heat at varying dryness (steam pressure 0.2 MpaG)
|
This table shows that the latent heat of the steam at 25% dryness is only 25% of the steam at 100% dryness. This means that four times more of this steam is equivalent to high quality steam at 100% dryness. As you can see, the dryness is an important factor for the latent heat.
The recent models of boilers generate superheated steam by heating dry saturated steam using a superheater operated with recycled exhaust heat. If the boiler supplies this steam, heat loss during the transport will only reduce the temperature of superheated steam and will not affect the latent heat of condensation during heating.
In addition, as explained in the previous volume, reducing the steam pressure at the boiler outlet will increase the dryness of steam by taking advantages of superheating performance at a throttle.
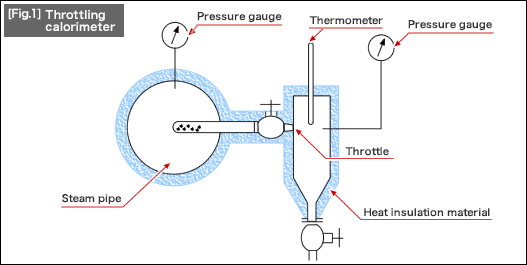
The dryness of steam, which is an important factor for boilers, can be measured by using a throttling calorimeter as shown in [Fig.1]. Just like the figure, insert a tube with holes into the steam pipe and measure the temperature and pressure of the steam passing through the throttle. Based on these measured values, you can find out the heat quantity and compute the dryness X using the steam table. As you can see, the heat retention control for steam piping is extremely important during steam transport in terms of the dryness control as well.
Heat insulation tubes made of glass wool are generally selected for heat retention control of steam piping. Adopting a thicker heat insulation layer can improve heat insulation, but it will be more costly. As a middle ground between the cost and effect, heat-insulation materials of 25 to 100 mm thickness are commonly used. Realistically, you cannot reduce the piping heat loss to zero. It is also important to remove drained water generated in the pipes.
![[Fig.1] Transition of water by heating [Fig.1] Transition of water by heating](/tt/en/surface/279_1.gif)
![[Fig.2] Three states of water, sensible heat & latent heat [Fig.2] Three states of water, sensible heat & latent heat](/tt/en/surface/279_2.gif)
![[Fig.3] Saturated water, wet steam, and superheated steam [Fig.3] Saturated water, wet steam, and superheated steam](/tt/en/surface/279_3.gif)
