|
Electrodeposition coatings can be can be broadly classified into cationic and anionic types. As shown in [Fig.1], the subject item (workpiece) to be painted for the cationic type is made into a cathode, and the counter electrode is made into the anode, and vise versa for the anionic type. When the paint subject is fully submerged and a DC current is applied from a rectifier, ionic paint particles precipitate by electrophoresis. The precipitated paint particles lose ionic nature and become insoluble forming nonconductive film resistance. Therefore, thin film coating is possible even on paint subjects with complex shapes. The Cathodic Electrodeposition Painting does not cause the base metals of the paint subjects to discolor due to oxidation since the subjects become cathodic. But with the Anionic Electrodeposition Painting, such reaction occurs, and therefore, it not used to paint copper, brass, or silver plated objects. For the electrodeposited film to grow thicker, the film needs to be conductive. But when the current flows, resin particles on the paint subject surface precipitate immediately, and form a nonconductive film and fuse due to joule heating. The insulating film should not grow thicker any further, but it actually does. It is supposed that gases from electrolysis keeping the surface porous, preventing formation of nonconductive surface film. |
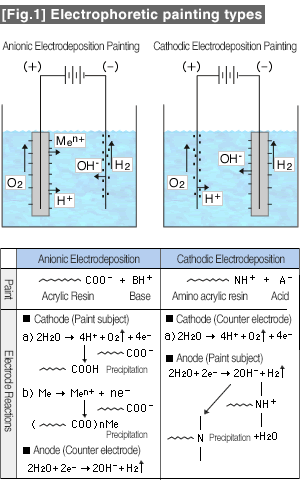 |
The porous coating film would melt while curing and becomes a continuous film, acquiring excellent anti-rusting effects. Comparisons between the anionic and the cathodic electrodeposition paintings in the [Table 1].
[Table 1] Comparisons between the anionic and the cathodic electrodeposition paintings
|
||||||||||||||||||||||||||
