"Hexavalent chromium" is designated as harmful substances by Japanese laws related to environmental conservation, such as Water Pollution Control Act, Soil Contamination Countermeasures Act, as well as those related to industrial health and safety. These laws and legislations strictly regulate its application and discharge methods along with the threshold limit value for discharge.
In order to comply with the restriction of the use of certain hazardous substances in electrical and electronic equipment (RoHS) to be enforced in July 2006, the hexavalent chromium content (ppm) is a serious problem for rubber, plastic, paint, chemical film, and ceramic products.
Hexavalent chromium
What is hexavalent chromium? Chromium is a metal element with atomic number 24, melting point of 1,905°C, and specific weight of 7.1 g. Because many of its compounds are colored, the name was derived from "chroma (Greek word meaning 'color')".
In addition to being used as decorative and industrial (hard) chrome plating, chromium is used in metal alloys, such as high-speed steel, stainless steel, nichrome, and KS magnet steel.
Chromium metal is not water-soluble. However, chromium compounds are relatively soluble in water. Chromium in chromium compounds is a positive ion carrying two to six positive charges. The number of electrical charges varies by compounds.
In the case of chromium plating solution, for example, chromic acid (H2CrO4) is generated by dissolving commercially available chromic anhydride into water. The reaction occurs as follows:
CrO3 + H2O → H2CrO4
Chromic acid is dissociated into hydrogen ion and chromate ion in the solution.
H2CrO2 → 2H+ + CrO42-
Direct current electrolysis produces hydrogen gas (H2) at the cathode. Chromate ion (CrO42-) will be attracted to the anode and turned into hexavalent chromium ion (Cr6+) and oxygen (O2) by a catalytic action at the anode. Hexavalent chromium becomes metallic chromium after receiving electrons in stages.
Cr6+ + 3e- → Cr3+, Cr3+ + 3e- → Cr
As we have seen, hexavalent chromium serves an important role as a supply source of chromium metal in plating solution. However, metallic chromium, which is used for plating films, does not contain hexavalent chromium.
Now, let's look at the chromate treatment after applying zinc plating. Electro-galvanizing and hot-dip galvanizing are used for construction materials, structures, ship equipment and more to prevent corrosion. However, zinc is known to form zinc oxide (white rust) by reacting with oxygen in the air.
To minimize this unfavorable character, the chromate treatment came in handy. The chromate treatment is a process where galvanized products are dipped into chromate treating solution, consisting primarily of chromic acid, to form an anticorrosive chromate film (such as CrO3, Cr2O3, and nH2O) containing hexavalent chromium on the plating surface. The higher content of hexavalent chromium in a film results in superior anticorrosive properties. Even if the chromate film has been damaged for some reason, the surrounding hexavalent chromium works great in repairing its property on its own.
There are various types of chromate films: bright chromate, colored chromate, black chromate, green chromate, and more. The content of hexavalent chromium differs for each of them. In general, it should be in the range between 20 and 120 mg/m2.
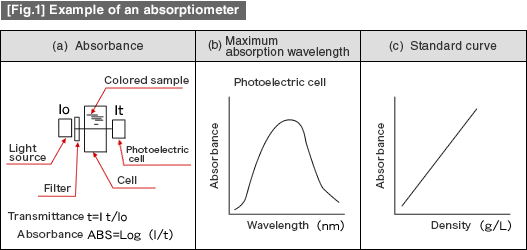
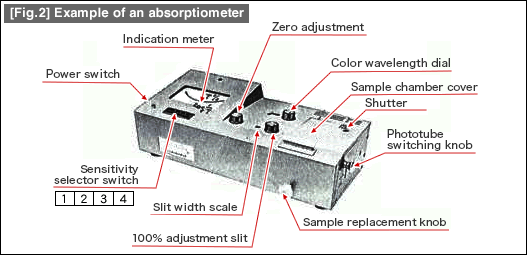
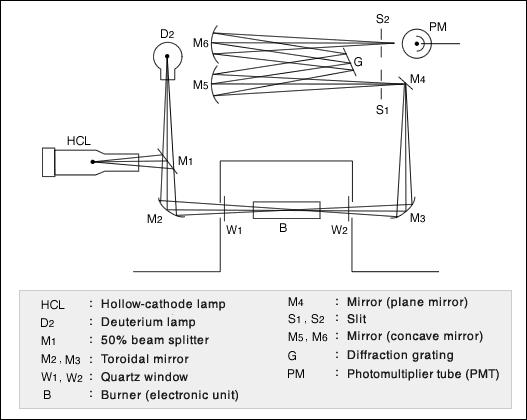
![[Fig.1] Example of ICP discharge tube (torch) [Fig.1] Example of ICP discharge tube (torch)](/tt/en/surface/236_1.gif)
![[Fig.2] Example of ICP emission analysis system [Fig.2] Example of ICP emission analysis system](/tt/en/surface/236_2.gif)