There are wide variety of anodize layer types with varying characteristics for aluminum and aluminum alloys.
The factors that determine the surface layers depend on the base metal. The mechanism of anodized layer forming differs from the electro-plating where anodized layers are created by the base metal being electrolytically dissolved to form oxidized layers. The thicker the layer becomes, more base metal is dissolved. Additionally, the base metal properties dictate how easily the oxidized layers can be formed.
There are many types of aluminum alloys with various compositions depending on their purposes, and they can be divided into heat treated alloys and non heat treated alloys. From a machining perspective, there are plates, bars, rods that are rolled, and cast, and die-cast mateials.
Various levels of anodizing difficulties based on different alloys exist and a number of schemes have been developed for the difficult alloys. Generally practiced anodizing processes are shown on [Table 1] below.
[Table 1] Types of Anodize Processes
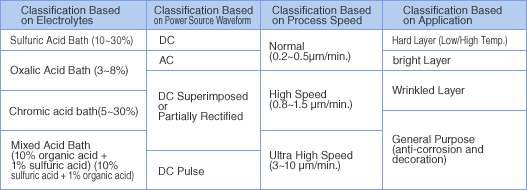
There are virtually infinite number of industrial anodizing processes combinations as the [Table 1] suggests, based on bath types, power supply waveforms, process speeds, and application purposes.
Currently, the most common electrolyte type is the sulfuric acid bath. The reason for the popularity is that the formed layer is colorless, and the power supply voltage and power consumption required for the electrolysis is low and economical for colored parts production. For specialized colors and wear resistance requirements, oxalic acid bath layers and oxalic acid added sulfuric acid bath are used.
Of the various electrolytes, the chromic acid bath is used almost only in the aerospace industry. As for the classification based on power supply waveforms, AC, AC superimposed, and pulse methods are used when the orthodox continuous DC does not yield satisfactory results.
Process speed classification in turn is a classification based on electrical current density. Large applied currents will form hard oxide layers by preventing the formed layer from dissolving into the electrolytes.
Various anodizing types discussed so far are in commercial use based on application requirements. Various aluminum materials will be discussed in the next volume.