The primary purposes of plating on plastic materials were to add decorative elements such as luxurious feel achieved by the metal-textured surface, as well as to prevent degradation of the plastic resin.
For the automobile components, polyacetal was used in place of zinc die cast, while nylon and polycarbonate resin were adopted in place of steel.
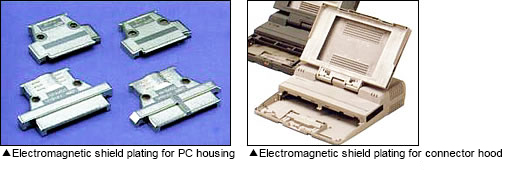
In today's IT era, electroless copper/nickel plating is applied on polycarbonate computer housings, keyboards, connector hoods and more, in order to protect these devices from electromagnetic waves.
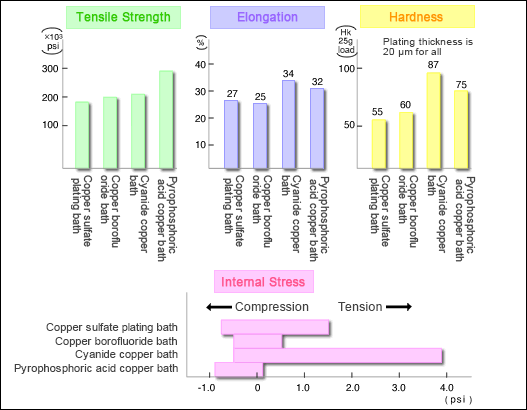
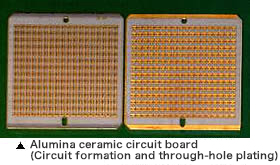
Examples of the electroplating application also include circuit boards, connectors, and IC testers that are made of heat-resistant special engineering plastics or super engineering plastics, adding superior bonding and soldering properties.In this case, the plating adhesion must be high.The general process of plating on plastic materials is as follows:
Degreasing -> Etching -> Catalyzing -> Electroless Plating -> Electroplating
The etching process corrodes the plastic surface and selectively dissolves out butadiene using concentrated chromium acid or sulfuric acid to prepare the material for the next process where strong metal catalysts are formed.
Catalyzing is a process in which catalytic nuclei (Au, Ag, Pt, Pd, etc.) necessary for the next electroless plating are deposited onto the plated material.
The electroless plating is designed to add conductivity to plastic materials by applying copper or nickel plating over them, using electroless plating solution containing chemical reducing agent, which acts as electricity used in electroplating.This process prepares the material for the next electroplating step.