Crevice Corrosion occurs with steel and other metals, as well as with passive surface layer forming metals such as stainless steel, aluminum alloys, and titanium alloys.
Compounded metal structures contain numerous clearances (crevices) where similar and dissimilar metal members and materials are jointed, as well as under scales and corrosion byproducts and coatings.
The crevices in discussion here are unlike the ones in typical discussions but are very small, in order of 1/100mm scale. Even into these small crevices, water and other liquids will intrude. Once such liquids intrude into these clearances, they would stay there since there would be little exchange with the outside and the oxygen concentrations become low. This oxygen concentration differentials cause formation of differential aeration batteries, and the crevices low in oxygen concentration will corrode. [Fig.1] illustrates an example of crevice formation on a metal plate with a material in contact.
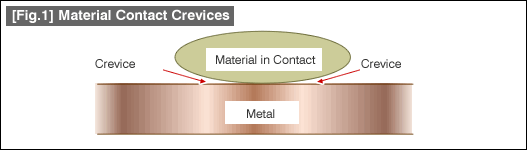
Anodic reaction in crevices, and cathodic reaction on the external surfaces will progress. The metal ions generated by the anodic reaction will become of corrosion byproduct. With crevice corrosion of stainless steel equipment, potential difference between the equipment body and crevices may be as high as 0.2V (200mV) or more.
As a reference, corrosion potential of metals and alloys in sea water is shown in [Table 1]. The metals in the upper positions are more susceptible to corrode than the ones in the lower positions. This hierarchy is called Corrosion Potential Series.
These potentials are referenced by comparative SCE (saturated calomel electrode). The stainless steel (passive) is with a passive surface layer, and stainless steel (active) is without the passive surface layer.
[Table 1.] Corrosion Potential of Metals in Sea Water (V)
|
![[Fig.1] Pitting Corrosion Mechanism](http://www.misumi-techcentral.com/tt/en/surface/images/056.gif)
![[Fig.1] Differential Aeration Battery](http://www.misumi-techcentral.com/tt/en/surface/images/055_1.gif)
![[Fig.2] Differential Aeration Battery condition with a Stake](http://www.misumi-techcentral.com/tt/en/surface/images/055_2.gif)
![[Fig. 1]](http://www.misumi-techcentral.com/tt/en/surface/images/054.gif)
