For plating products with hydrogen embrittlement risks, baking processes are performed as means of dehydrogenation process. The baking process typically involves heating the products in furnaces at 190~220 deg.C to purge the hydrogen. The heating time will depend on the plating type, pre-plating process used, deposition thickness, alloy type, and the condition of the base metal.
(1)Pre-process and effects of baking
[Fig. 1] and [Fig. 2] below show the effects of dehydrogenation by baking on material samples with zinc plating where one sample with no hydrogen occlusion, and the other with hydrogen occlusion during the pre-process. Zinc plating is used as a representative more likely to cause hydrogen embrittlement.
[Fig. 1] shows zinc plated samples pre-processed in a non hydrogen occluding acid bath pickling. The sample does occlude some hydrogen through the zinc deposition layer but one hour of baking will effectively dehydrogenate the sample in all types of zinc baths as seen.
![[Fig. 1] Non hydrogen occluding pickling used [Fig. 1] Non hydrogen occluding pickling used](http://www.misumi-techcentral.com/tt/en/surface/images/009_01.gif)
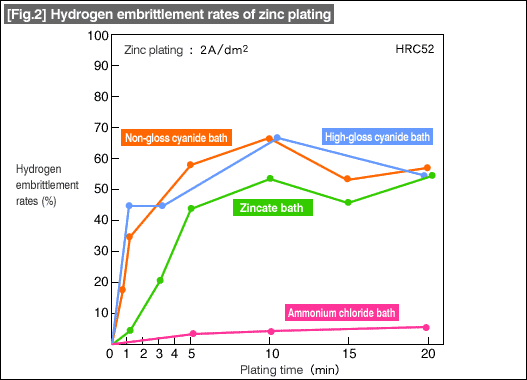
[Fig.2] shows samples that occluded large amounts of hydrogen during the pre-process pickling as well as the zinc plating process. As can be seen in the graph, little hydrogen is purged from the alloy even after long baking time.
![[Fig. 2] Hydrogen occluding pickling used [Fig. 2] Hydrogen occluding pickling used](http://www.misumi-techcentral.com/tt/en/surface/images/009_02.gif)
From the above, it can be understood that prevention of hydrogen occlusion is important during the pickling process, or some type of dehydrogenation process will be required before the baking process if the work had already occluded some hydrogen during pickling. Effective hydrogen occlusion prevention includes use of proper inhibitors, and in the latter case, immersion in high temperature alkaline degreasing bath. As can be seen in the graphs, ammonium chloride zinc bath is effective in preventing hydrogen embrittlement.
![[Fig.1] Hydrogen embrittlement rates of various plating processes [Fig.1] Hydrogen embrittlement rates of various plating processes](http://www.misumi-techcentral.com/tt/en/surface/images/008_1.gif)
![[Fig.1] Hydrogen embrittlement rate and sitting-out time duration](http://www.misumi-techcentral.com/tt/en/surface/images/007_01.gif)
![[Fig. 2] Alkaline wash after pickling and hydrogen embrittlement](http://www.misumi-techcentral.com/tt/en/surface/images/007_02.gif)
![[Fig.1]Action of the inhibitor [Fig.1]Action of the inhibitor](http://www.misumi-techcentral.com/tt/en/surface/images/006_1.gif)
