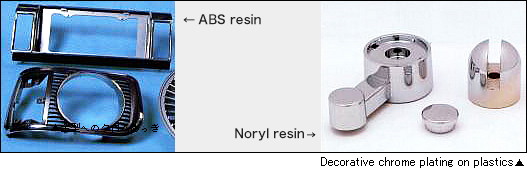
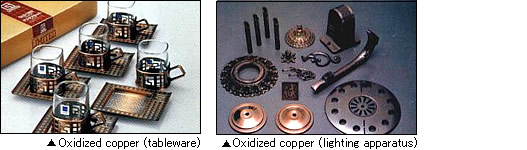
Electroless nickel-phosphorus (5 to 13%) alloy plating is the typical electroless plating most frequently used in industrial processes. The demand is increasing due to the excellence in mechanical, electrical, and physical properties while the uniform film thickness can be obtained even on a complex shape.
The table shown below lists the application examples and purposes of electroless nickel-phosphorus alloy plating.
[Table] Application examples and purposes of electroless nickel-phosphorus alloy plating
|
One of the characteristics of electroless nickel plating is that the hardness increases by applying heat treatment after plating. The hardness of Hv500 to 550 right after plating becomes around Hv800 and 1000 by treating with heat at 400℃.
Although electroless nickel plating can be applied on most of the metals, plastics, and ceramics, the plating will not be deposited on particular metals, including tin, lead, zinc, cadmium, or antimony. This is because the electroless nickel plating inhibits the catalytic action at metal deposition.
In addition to nickel-phosphorus plating, nickel-boron (1% of boron) alloy is also used for electroless nickel plating. This material is more expensive, but it is the preferred choice for electrical components because of its remarkable solderability and heat resistance performance.