Surface treatment and environmental conservation
Background of environmental conservation
In the past, metal surface treatment plants including electroplating factories were exposed to criticism that they were responsible for pollution problems resulting from cyanogen or hexavalent chromium. This had forced the management to make a substantial investment out of their scarce monetary resources in preventing such pollution, which caused them to bear with the high running cost.
The Water Pollution Control Act must be enforced for wet surface treatments as it involves water and its drainage. The Poisonous and Hazardous Substances Control Law applies to processes that involve use of acid/alkali, poisonous, or deleterious substances. In addition, the "effluent standards" must be observed for water discharged into public waters. The Sewerage Act applies to water discharged into sewers. Such drainage must be compliant with the "effluent standards" defined by the Sewerage Code of the corresponding district.
If it involves a process that generates harmful gas or mist, the concentration measurement and voluntary inspection are required for the department in charge of the process according to the Industrial Safety and Health Act. This includes an installation of purification systems or adopting an operation management process.
Boilers used for drying plating solution or products as well as bake finish of paints are regulated by the exhaust emission standards under the Air Pollution Control Act.
Each local government has its own "antipollution ordinance" to control water quality, air, noise, vibration and so on.
More restrictions are added to these laws and regulations year after year. Since the initial enactment, the regulatory values have become stricter than ever.
Aside from the pollution problems we talked about so far, the world is also witnessing a deterioration of global environment in recent years, such as the disappearing ozone layer by CFCs used in refrigerant and spray cans, and global warming associated with the carbon dioxide increase resulting from fuel burning of automobiles and thermal power plants.
Various measures have been adopted for preventing further deterioration. One of them is called VOC regulations (VOC refers to Volatile Organic Compounds including organic solvents). Another example is the CO2 emission reduction policy as part of the energy-saving measures. In addition, more companies are now practicing "green procurement", which is a strategy of preventing environmental pollution by avoiding materials containing harmful substances like cadmium, lead, or mercury in the procurement process. These measures have been adopted not only in Japan but also on a worldwide scale with an exception of some countries.
In the European Union (EU) for example, they have enforced the RoHS Directive regulating the harmful substances contained in electrical and electronic devices together with the WEEE Directive regulating the industrial waste such as electrical products since the year 2007. These directives regulate the amount of organic/inorganic harmful substances in products.
The Soil Contamination Countermeasures Act was enacted in Japan to resolve soil contamination problems that occur frequently with redevelopment of old factory site into a residential area. This regulation not only applies to selling the property that was formerly used as factory site but also applies to cases where the land usage changes even when the owner remains the same. The process requires analysis of the soil to check for harmful substances and its concentration. If the soil is contaminated, the land is subject to soil purification.
Let's look into the details of these regulations from the next lecture.
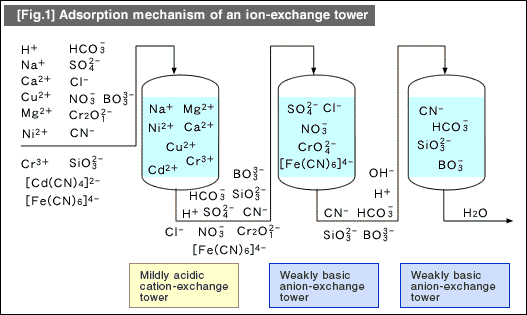
![[Fig.1] One cycle of ion-exchange resin [Fig.1] One cycle of ion-exchange resin](/tt/en/surface/254.gif)
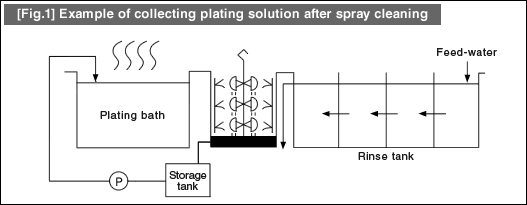
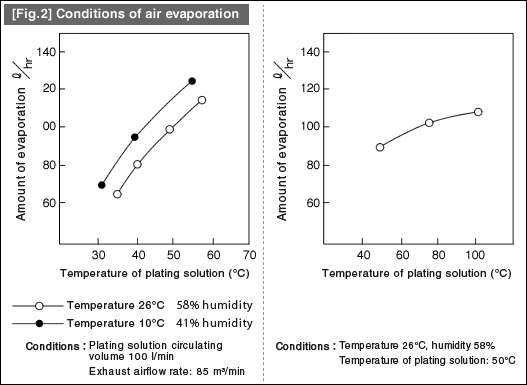
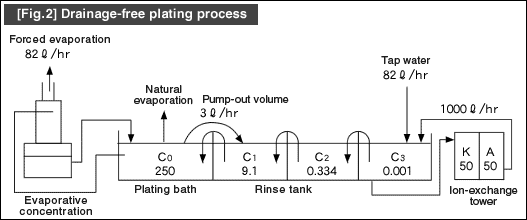
![[Fig.3] [Fig.2] [Fig.3] [Fig.3] [Fig.2] [Fig.3]](/tt/en/surface/252.gif)
