(3) Pickling process with other acids
The following table shows various hydrogen embrittlement rates of a steel alloy ([SK5] [W1-8] [1.1525_C80W1]) in varying acid bath solutions.
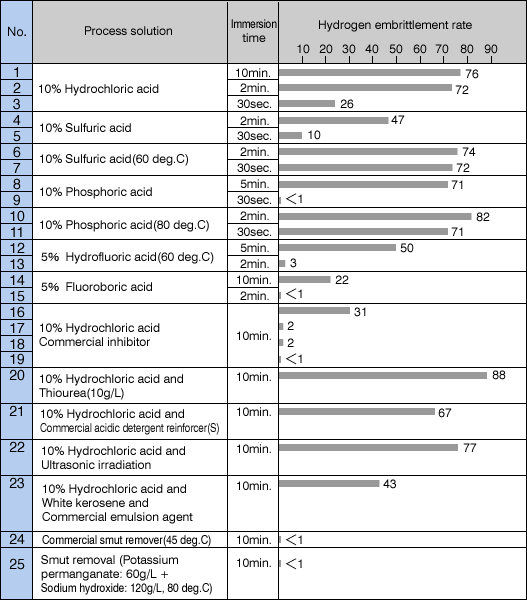
The use of hydrochloric acid has been mentioned before, and other acid bath pickling also shows similar results. Pickling with sulfuric acid is as common as with hydrochloric acid. Sulfuric acid shows low hydrogen embrittlement rates at lower temperatures but has low rust removing capability, thus it is typically used at around 60 deg.C. At this temperature, sulfuric acid shows hydrogen embrittlement rate similar to that of hydrochloric acid. Phosphoric acid is also similar. As seen in the table above, fluorine acids such as hydrofluoric acid, fluoroboric acid, and hydrochloric acid with commercial inhibitor shows low hydrogen embrittlement rates. And it can be recognized that de-smut bath used after pickling is also low in embrittlement rates.
(4)Electro rust removal and electro de-greasing, etc.
Rust removal, de-greasing, de-smutting, and activating by electrolysis are performed in acidic and alkaline solutions, after pickling processes. In these cases where products are processed by cathode electrolysis, hydrogen embrittlement occurs due to electrolysis of water. With anode electrolysis in alkali baths, very little embrittlement occurs. In acid bath, hydrogen embrittlement occurs during non-energized durations due to chemical dissolution.
![[Graph] [Graph]](http://www.misumi-techcentral.com/tt/en/surface/images/0004_1.gif)